SmartSuite for
Pharmaceuticals
SmartSuite unifies quality, regulatory, clinical, manufacturing, and operational workflows—replacing disconnected pharma systems with one modern, connected platform.

Transform Quality, Regulatory & Operational Workflows
Pharmaceutical organizations often manage critical processes across disconnected systems — from quality and regulatory tools to clinical and manufacturing platforms. These silos create data gaps, slow compliance activities, and increase operational risk.
SmartSuite replaces this fragmented environment with one connected platform that unifies teams, data, and workflows across the pharma value chain.
SmartSuite helps pharmaceutical organizations:
- Integrate quality, regulatory, clinical, manufacturing, and ops workflows
- Reduce system fragmentation across QMS, RIM, and operational tools
- Standardize workflows for audits, deviations, CAPA, and documentation
- Improve coordination between quality, regulatory, clinical, and operations
- Gain real-time visibility into compliance, risks, actions, and approvals
Integrated Quality Ops
Quality • Regulatory • Clinical • Manufacturing
Unified data + connected compliance workflows
Connected Programs
QMS • RIM • CAPA • Audits
One platform replacing fragmented pharma systems
Rapid Implementation
Prebuilt pharma solutions speed adoption
No-code tools adapt quickly across teams & sites
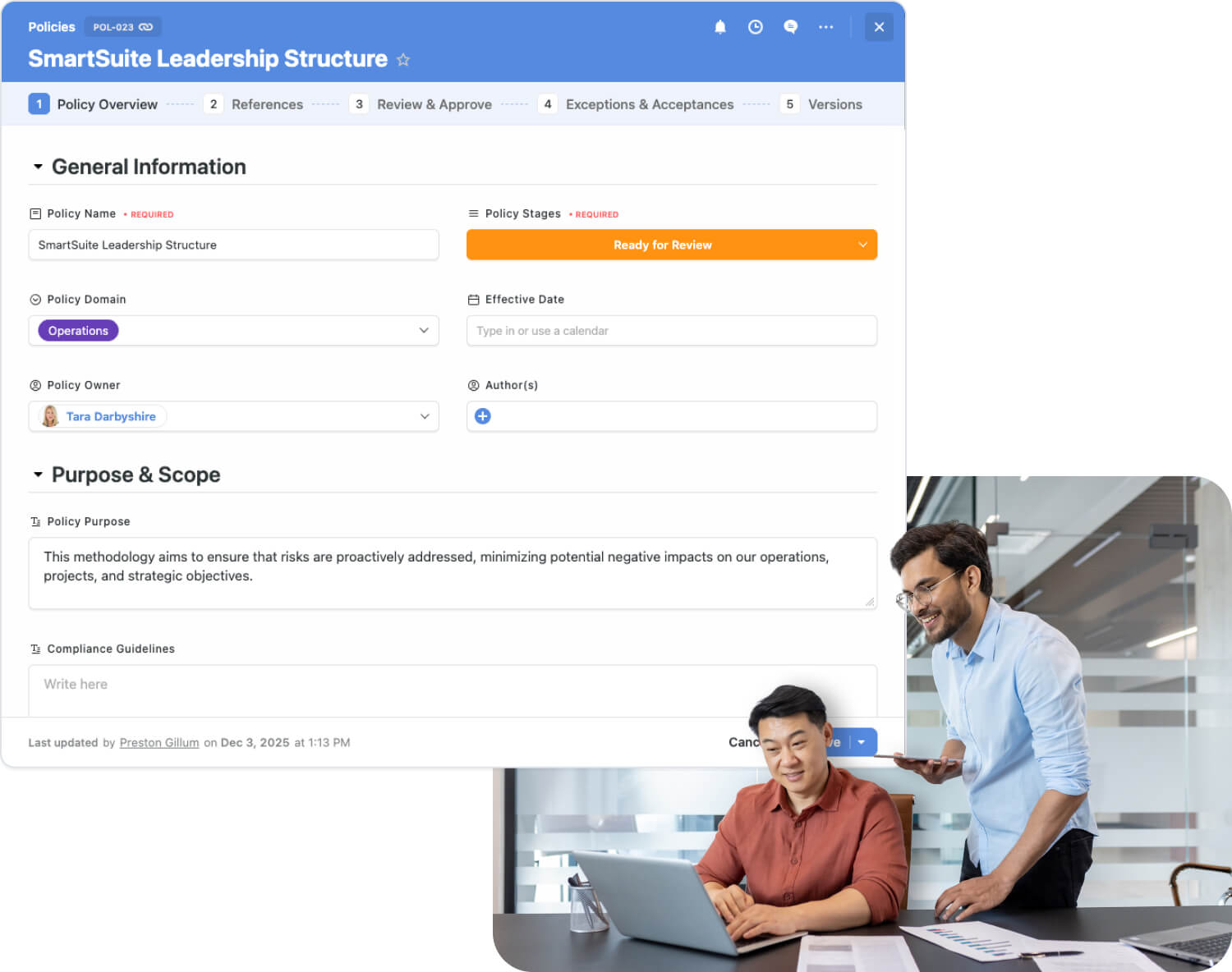
How SmartSuite Supports Pharmaceutical Organizations
SmartSuite helps pharmaceutical companies manage GxP, CAPA, and validation processes in one compliant platform. Simplify audits, enhance visibility, and maintain FDA and EMA readiness.
Maintain Continuous GxP Compliance
Centralize GxP compliance activities in a single validated workspace. SmartSuite standardizes how teams manage controls, documentation, and approvals—supporting ongoing compliance with global regulatory requirements.
Key capabilities include:
- Track GMP, GLP, GCP, FDA 21 CFR Part 11, and EMA Annex 11 requirements
- Maintain validated electronic records and electronic signatures
- Manage compliance documentation with version control and audit trails
- Monitor compliance status and readiness across sites in real time

Improve Product Quality and Audit Readiness
Manage quality events and corrective actions with full traceability. SmartSuite helps teams investigate deviations, implement CAPAs, and demonstrate effective quality oversight during inspections.
Key capabilities include:
- Capture deviations, complaints, and nonconformances centrally
- Perform root-cause analysis and manage CAPAs from issue to closure
- Track effectiveness checks and corrective action completion
- Link quality events directly to audits and regulatory reporting

Streamline Validation and Documentation Workflows
Digitize validation activities to reduce manual effort and inspection risk. SmartSuite ensures validation documentation, reviews, and approvals remain complete and inspection-ready.
Key capabilities include:
- Manage validation plans, protocols, and evidence digitally
- Automate document reviews, approvals, and change tracking
- Maintain a continuous validated state across systems and processes
- Support inspection readiness with centralized documentation

Ensure Supplier Compliance and Accountability
Gain visibility into supplier quality and compliance across the supply chain. SmartSuite helps pharmaceutical teams manage vendor certifications, audits, and performance proactively.
Key capabilities include:
- Track supplier certifications, audits, and quality agreements
- Assess supplier risk and performance using scoring and trends
- Manage corrective actions tied to supplier findings
- Maintain traceability between suppliers, products, and quality events

Featured Solution Suites For Pharmaceuticals
Explore SmartSuite Solution Suites built to ensure compliance, quality, and operational excellence in regulated pharma environments.
Manage non-conformances, CAPAs, and inspections with full traceability from issue detection to resolution.
Ensure adherence to AS9100, ITAR, DFARS, CMMC, and aerospace regulatory mandates with structured workflows and audit-ready reporting across programs and operations.
Plan, execute, and report on audits with complete assurance oversight — linking findings to risks, controls, and remediation actions in a single connected workspace.
Standardize vendor due diligence, centralize assessments, and monitor ongoing risk exposure to ensure supplier reliability and compliance.
Coordinate R&D and formulation projects with task dependencies, document control, and approval workflows that maintain traceability.
Automate training assignments, track completion by role, and manage SOP lifecycles with version control and electronic signatures.
Pharmaceuticals
Challenges
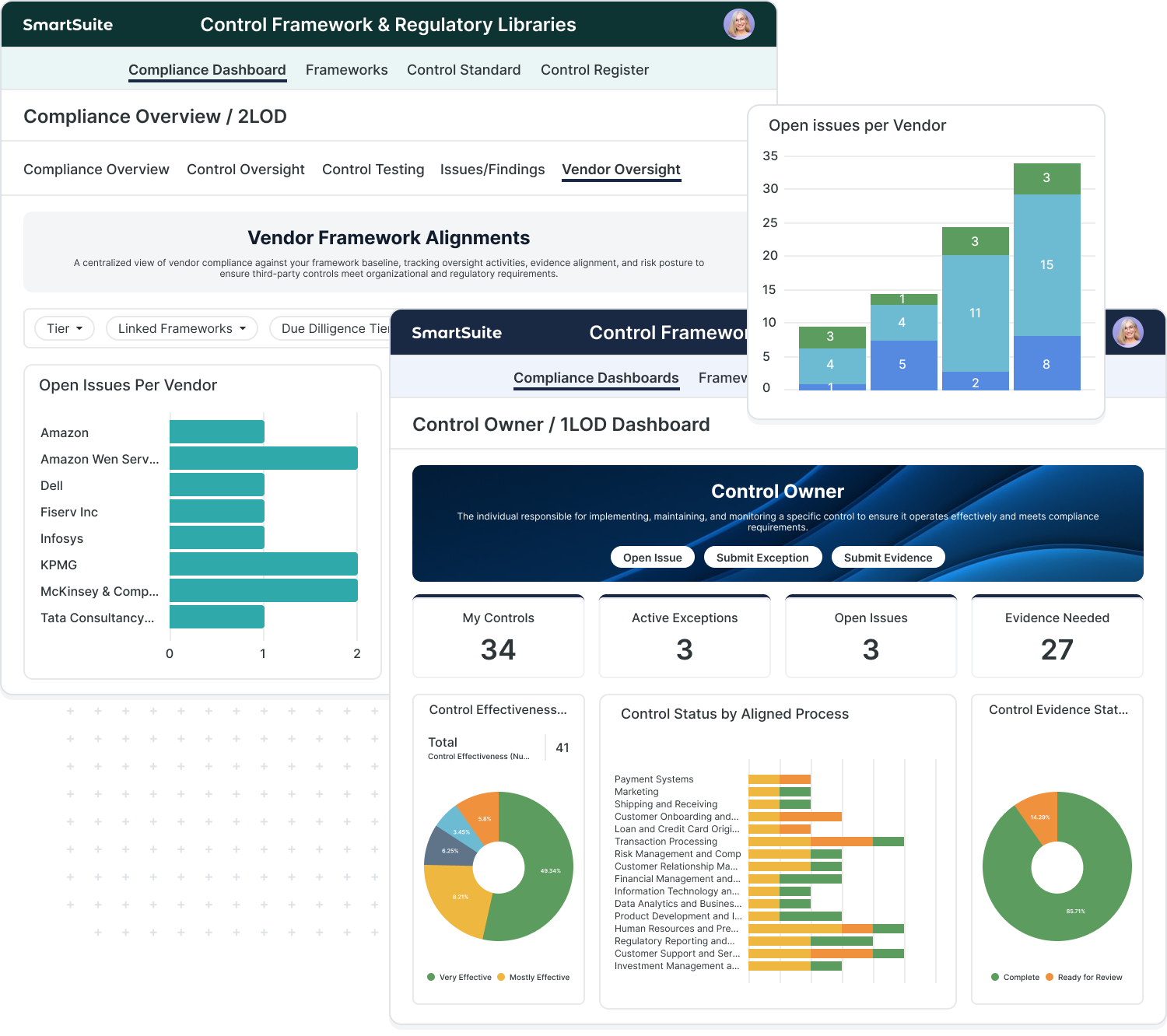
Why Smartsuite For Pharmaceutical Companies
GxP-Aligned Connected Workflows
Unify quality, regulatory, and R&D operations within one platform built for GMP, GLP, and GCP environments.
Designed for FDA and EMA Compliance
Pre-built templates aligned with 21 CFR Part 11, Annex 11, and ISO 9001 ensure traceability and audit readiness.
Validated Platform with Audit Trails
Every change is logged automatically, maintaining a compliant record of activity for audits and inspections.
AI-Powered Automation
Use SmartSuite AI Assist to summarize deviations, generate CAPA reports, or draft submission documents instantly.
Aligned with Regulatory Frameworks
Map controls and evidence to the frameworks your institution follows.
- FFIEC, CRI Profile, SOX, and OCC guidelines
- NIST and ISO cybersecurity standards
- Vendor oversight and audit traceability
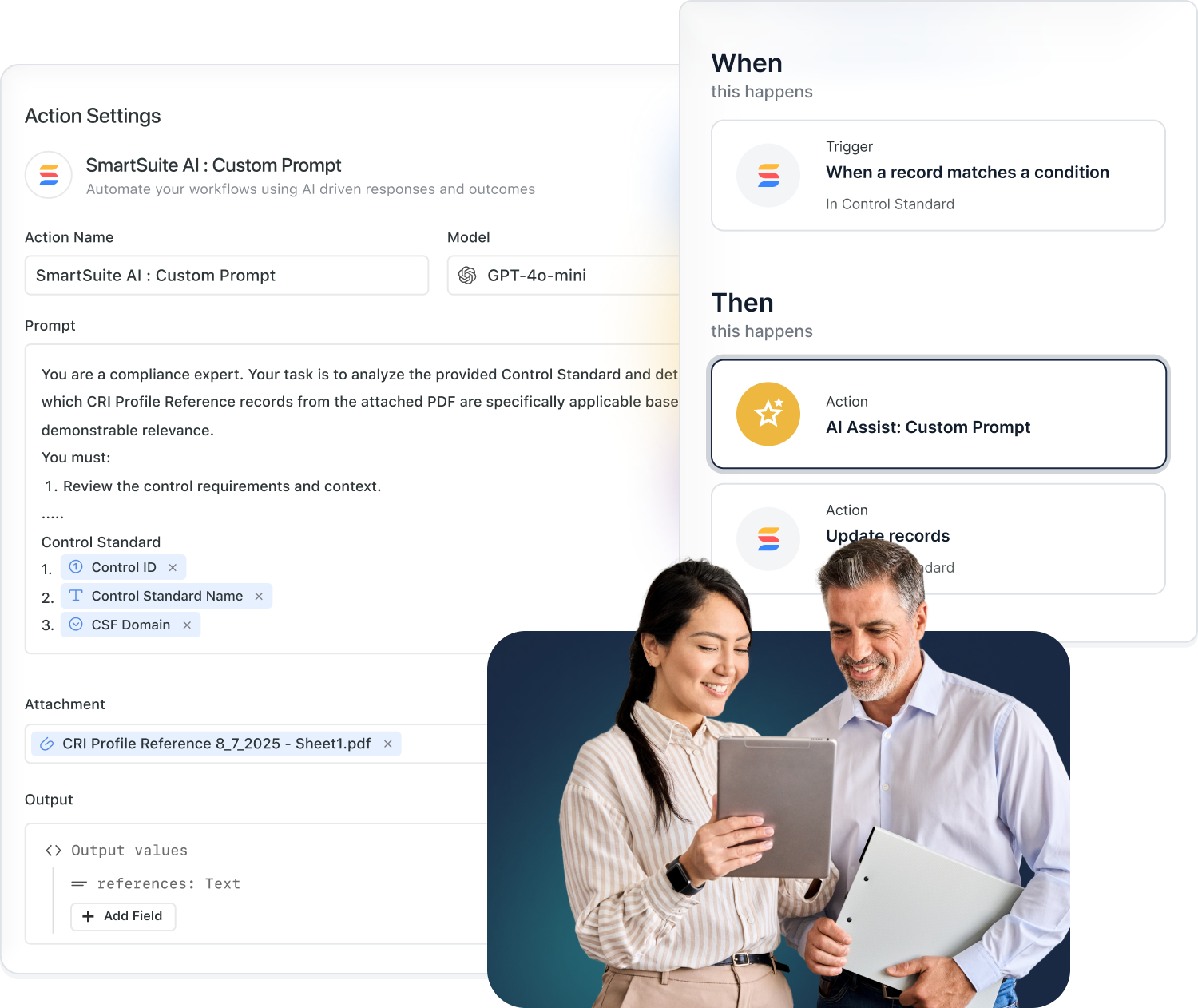
AI That Adapts to Your Industry — On Your Terms
SmartSuite embeds AI directly into your workflows to accelerate automation, reporting, and insight generation — while giving you complete control over which models you use and how your data is handled.
Choose from OpenAI, Anthropic, Google Gemini, AWS Bedrock, Azure, Perplexity, Nscale, IBM watsonx, and Xai. Connect your own API keys for full privacy and compliance.
- Bring Your Own AI
Accelerated first-response targets for high-severity issues, so critical incidents are acknowledged, triaged, and moving toward resolution as quickly as possible.
- AI in Action
Use AI to generate compliance reports, summarize audits, and build automations that enhance accuracy and efficiency.
- Data Privacy by Design
SmartSuite never uses customer data for model training. All AI calls route securely through your credentials.
- Customizable Intelligence
Mix models for different needs — reporting, content creation, risk analysis — and tailor performance per workflow.
A Unified Platform for People, Processes, and AI
SmartSuite unites people, data, AI, and systems on one open platform—designed to integrate with anything, secure everything, and adapt to any business process. From industry-specific workflows to enterprise integrations, SmartSuite gives every team the flexibility to work smarter together.
Explore Further
Read quick explainers on the ideas and best practices behind high-performing teams in your industry. See how SmartSuite turns critical workflows into connected, trackable work—so execution stays fast and reliable.
Featured Resources
Explore resources and insights for compliance, risk, and quality leaders in aerospace.
Frequently Asked Questions
Common questions from pharmaceutical quality, regulatory, and compliance teams.
SmartSuite maintains full audit trails, access controls, and electronic signatures, ensuring compliance with FDA and EMA data integrity regulations.
Yes. SmartSuite provides a validated framework with documentation for IQ/OQ/PQ and supports customer-specific validation processes.
Yes. SmartSuite connects suppliers, quality events, and manufacturing sites through integrated CAPA and audit management workflows.
Most organizations deploy within 60–90 days, depending on validation scope and integration requirements.
Ready to Connect Quality, Compliance, and R&D?
See how pharmaceutical leaders use SmartSuite to ensure GxP compliance, accelerate product development, and streamline audits.





