What Is 21 CFR Part 11 Compliance?
21 CFR Part 11 compliance is a critical regulation for organizations operating in life sciences industries.
This regulation, established by the U.S. Food and Drug Administration (FDA), is essential for ensuring the integrity and authenticity of electronic records and electronic signatures.
In this detailed guide, we delve into the nuances of 21 CFR Part 11, exploring its requirements, benefits, and how SmartSuite can assist organizations in achieving compliance.
TL;DR
- 21 CFR Part 11 regulates the use of electronic records and electronic signatures for FDA-regulated organizations, ensuring data integrity, authenticity, and legal compliance.
- Key requirements include secure electronic records, unique electronic signatures linked to records, audit trails, and SOP-driven processes.
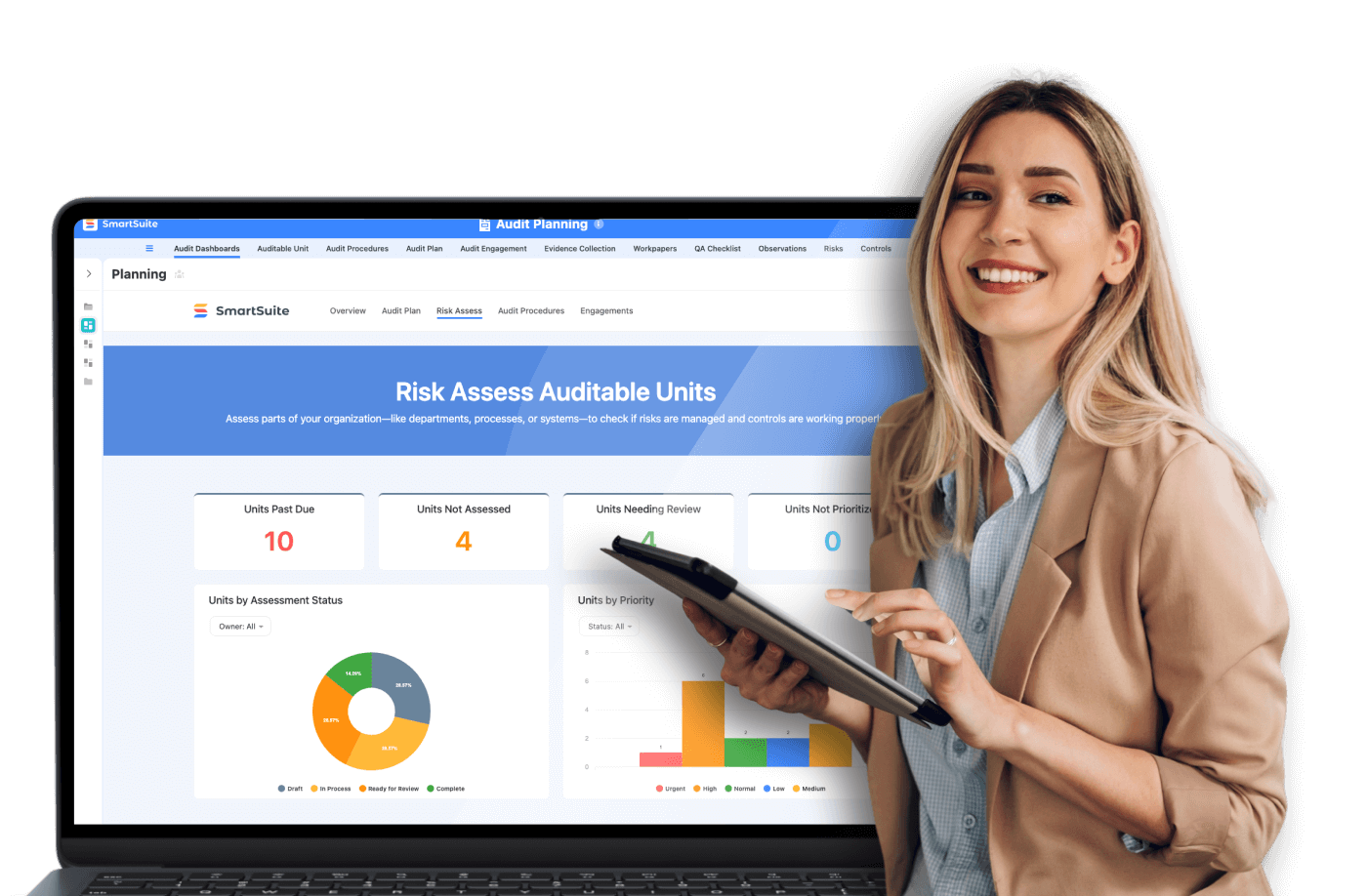
- SmartSuite supports compliance by offering customizable workflows, centralized document storage, audit trails, and automated processes, helping life sciences organizations maintain regulatory adherence, operational efficiency, and data security.
What is 21 CFR Part 11?
21 CFR Part 11 refers to Part 11 of Title 21 of the Code of Federal Regulations. These regulations set forth the criteria under which the FDA considers electronic records and electronic signatures to be trustworthy, reliable, and equivalent to paper records.
Key Sections of 21 CFR Part 11
- Subpart A - General Provisions: Outlines the scope and applicability of the regulations.
- Subpart B - Electronic Records: Establishes requirements for the use of electronic records in place of paper records.
- Subpart C - Electronic Signatures: Details requirements for the integrity and use of electronic signatures.
Importance of Compliance
Regulatory Compliance: Ensures adherence to legal standards and mitigates risk of non-compliance penalties.
Data Integrity and Confidentiality: Protects sensitive data from unauthorized access and ensures the accuracy of records.
Operational Efficiency: Streamlines processes with digitized record keeping and reduces manual paperwork.
Who Needs to Comply?
Any organization under the purview of the FDA, notably those within:
- Pharmaceuticals
- Biotechnology
- Medical Devices
These organizations depend on comprehensive work management solutions like SmartSuite to align their procedures with compliance standards.
Core Requirements of 21 CFR Part 11
Electronic Records
Organizations must implement systems that ensure:
- Accessibility: Ensure that records can be accessed readily and are easy to retrieve.
- Accuracy: Maintain precise and reliable data without unauthorized alterations.
- Authenticity: Implement checks and balances to verify the origin and authorship of records.
Electronic Signatures
The regulation mandates that electronic signatures must be:
- Unique to each user
- Linked to their corresponding records
- Able to discern invalid or altered entries
Strategies for Achieving Compliance
Implementing SOPs
Develop and maintain Standard Operating Procedures (SOPs) tailored to ensure compliance. SOPs should outline security measures, access controls, and audit trail processes.
Utilizing Work Management Platforms
SmartSuite's platform delivers robust solutions for maintaining compliance through efficient workflow management, secure data handling, and detailed audit trails.
Features of SmartSuite for Compliance
- Customizable Workflows: Tailors processes according to compliance needs.
- Centralized Document Storage: Keeps all records secure and easily accessible.
- Audit Trails: Offers detailed logs of all interactions with records to ensure accountability.
Regular Training and Audits
Conduct routine audits and provide ongoing training sessions for staff to remain up-to-date with compliance protocols and enhance data security awareness.
Benefits of 21 CFR Part 11 Compliance
Enhancing Product Quality
Compliant systems lead to improved quality and safety of products, contributing to better consumer trust and market standing.
Facilitating Market Access
Compliance aids in navigating complex regulatory environments, facilitating smoother market entry and product approvals.
Cost Efficiency
Reduced reliance on paper-based processes translates into lower operational costs and improved resource allocation.
Challenges in 21 CFR Part 11 Compliance
Organizations may encounter challenges such as:
- Technological Limitations: Inadequate IT infrastructure can impede compliance.
- Integration Complexity: Difficulties in integrating compliance systems with existing processes.
- Resource Allocation: Necessity for significant investment in training and technology upgrades.
Conclusion: Partnering with SmartSuite for Success
Achieving 21 CFR Part 11 compliance is not just about meeting regulatory demands; it is about enhancing operational processes and safeguarding data integrity.
SmartSuite offers a comprehensive work management solution tailored to meet the complex needs of life sciences entities, providing robust features to ensure seamless compliance.
By aligning your compliance strategy with SmartSuite's innovative platform, your organization can not only meet but exceed regulatory expectations, positioning itself as a leader in quality and safety.
For more information on how SmartSuite can support your compliance journey, contact our team today.
Takeaway: Understanding and implementing 21 CFR Part 11 compliance is essential for maintaining regulatory alignment, improving operational efficiency, and ensuring data integrity. Equip your organization with SmartSuite's solutions to confidently navigate the regulatory landscape.
Get started with SmartSuite Governance, Risk, and Compliance
Manage risk and resilience in real time with ServiceNow.



