What Is CAPA Management in a Pharmaceutical Quality System?
Corrective and Preventive Action (CAPA) management is an essential component in pharmaceutical quality systems.
CAPA processes can significantly impact the overall efficiency and safety of pharmaceutical operations. As regulatory bodies continue to tighten their scrutiny on drug safety and manufacturing practices, effective CAPA management has become integral to meeting compliance and enhancing quality performance in the industry.
This article explores CAPA management, its importance, methodologies, and how SmartSuite can streamline these processes to optimize quality outcomes.
TL;DR
- CAPA ensures quality and compliance: Corrective and Preventive Actions help pharmaceutical companies detect, address, and prevent issues, meeting regulatory requirements and improving product quality.
- Structured CAPA process: Effective CAPA involves identification, evaluation, investigation, action planning, implementation, and reviewing effectiveness to drive continuous improvement.
- Technology streamlines management: Tools like SmartSuite automate workflows, centralize data, provide dashboards, and monitor compliance, making CAPA processes more efficient and reliable.
CAPA in Pharmaceuticals
What is CAPA?
CAPA stands for Corrective and Preventive Actions. In essence, CAPA is a systematic approach to detect, investigate, and correct systemic issues to prevent recurrence. The concept is deeply rooted in quality management systems and is a critical component of Good Manufacturing Practices (GMP).
The Two Pillars: Corrective and Preventive Actions
- Corrective Actions (CA): These are steps taken to eliminate the causes of a detected nonconformity or other undesirable situation.
- Preventive Actions (PA): These include measures implemented to eliminate the cause of potential nonconformities to prevent their occurrence.
Importance of CAPA in Pharmaceutical Quality Systems
Ensuring Compliance
Regulatory bodies such as the FDA require every pharmaceutical company to maintain a robust CAPA system. Failing to comply can lead to significant legal repercussions, including product recalls and fines. Having a well-structured CAPA system not only helps in maintaining compliance but also enhances the overall quality of products.
Enhancing Quality Control
CAPA processes help identify potential risks before they translate into real issues. By addressing these early, pharmaceutical companies can improve their product quality, thereby increasing consumer trust and market longevity.
Promoting Continuous Improvement
Implementing CAPA encourages a culture of continuous improvement within organizations. It ensures that lessons learned are effectively utilized to better processes, reducing downtime and increasing output quality.
Key Components of CAPA Management
Stage 1: Identification
Identification is the cornerstone of a CAPA management system. This involves detecting deviations, nonconformities, or undesirable situations that could potentially impact product quality.
Stage 2: Evaluation
Once identified, it is crucial to evaluate the scope and impact of the issue. This assessment helps prioritize CAPA activities ensuring resources are allocated efficiently.
Stage 3: Investigation
Conduct a root cause analysis to determine why the nonconformity occurred. Various methodologies, such as the "5 Why" analysis and Fishbone diagrams, can be effective here.
Stage 4: Action Plan
Based on the investigation, develop a detailed action plan. This plan should outline the corrective and preventive measures to be implemented.
Stage 5: Implementation
Execute the action plan. It is vital that this stage is meticulously documented to ensure all actions are trackable and verifiable.
Stage 6: Review and Effectiveness Check
Finally, review the outcome of the CAPA actions to ensure nonconformities are resolved and performance is improved. Adjustments may be necessary based on findings from the effectiveness check.
SmartSuite's Role in Optimizing CAPA Management
SmartSuite offers powerful tools and features designed to streamline CAPA processes and ensure organizations maintain high compliance standards.
Integrated Workflow Automation
SmartSuite's workflow automation capabilities allow pharmaceutical companies to automate routine CAPA tasks. This ensures timely actions and reduces the risk of human error.
Centralized Data Management
With SmartSuite, data related to CAPA initiatives can be stored in a centralized database, making access easy and efficient for team members across various departments. This functionality ensures data integrity and supports robust decision-making.
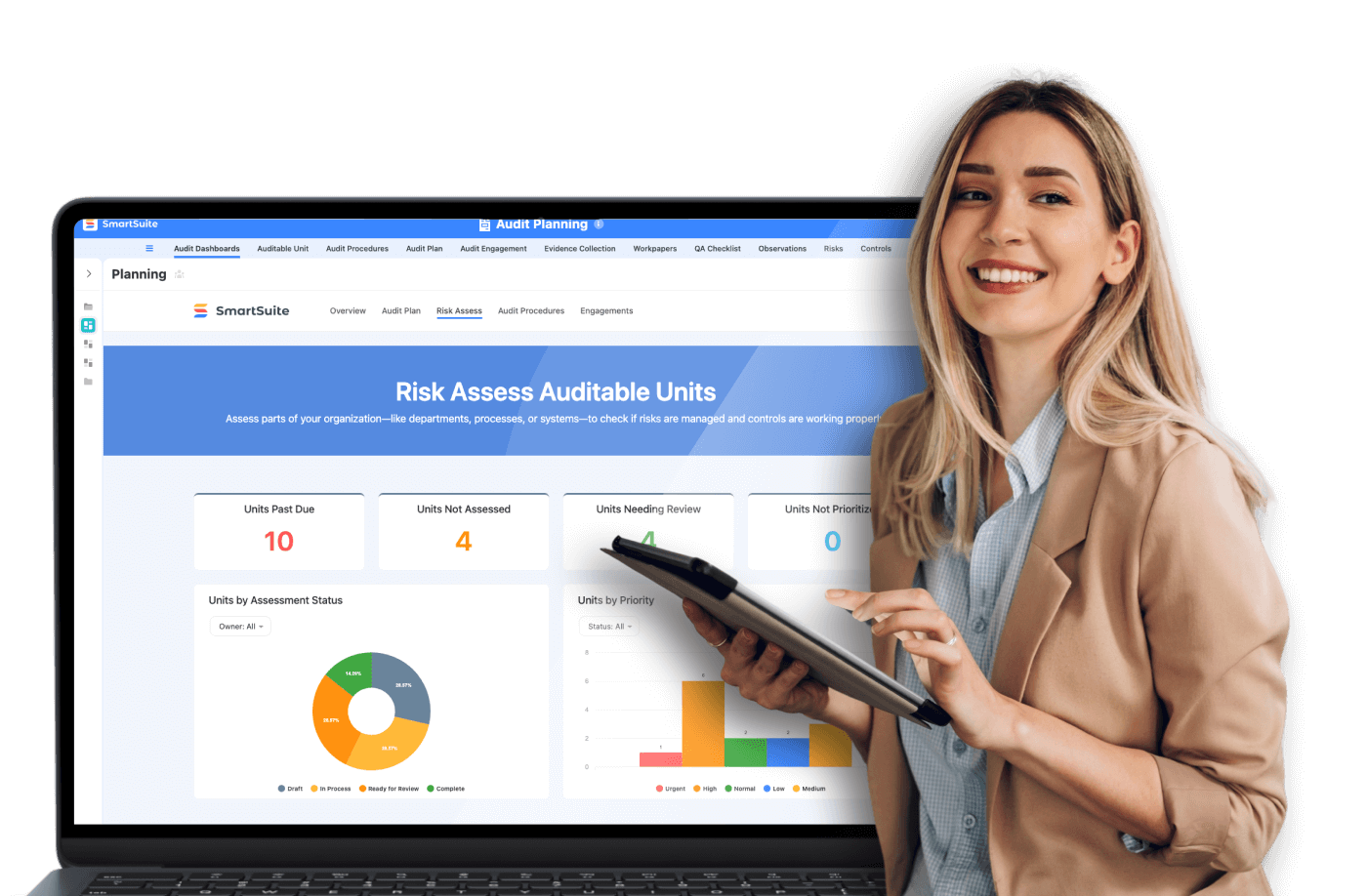
Customizable Dashboards
SmartSuite provides customizable dashboards that give managers a real-time overview of CAPA activities, enabling more proactive management and quicker response times.
Compliance Monitoring
SmartSuite’s compliance monitoring tools help in tracking compliance metrics and generating reports that are essential during audits. These tools aid in maintaining a current and comprehensive view of CAPA-related compliance activities.
Best Practices for Effective CAPA Management
Foster a Quality Culture
Encourage a culture of quality within your organization. Employees should be empowered and motivated to report issues promptly, ensuring quick identification and resolution.
Utilize Technology
Leverage solutions like SmartSuite to automate and optimize CAPA processes. Technology plays a crucial role in enhancing the efficiency and effectiveness of CAPA management.
Continuous Training
Regular training sessions and workshops ensure that team members are up-to-date with the latest CAPA methodologies and regulatory requirements.
Regular Audits
Schedule regular internal audits to assess the efficiency of the CAPA process. These audits help in identifying areas of improvement and ensure compliance with industry standards.
Challenges in CAPA Management and How to Overcome Them
Resistance to Change
Implementing a robust CAPA system may face resistance within organizations. Clear communication and demonstrations of the benefits can help overcome this challenge.
Data Management
Handling vast amounts of data required for CAPA can be challenging. SmartSuite provides the necessary infrastructure for efficient data management and ensures compliance.
Resource Allocation
Prioritizing resource allocation for CAPA activities is crucial. SmartSuite's analytics tools help in making data-informed decisions for optimal resource deployment.
Conclusion
CAPA management in a pharmaceutical quality system is pivotal for ensuring compliance, enhancing quality assurance, and fostering a culture of continuous improvement.
Though the process can be intricate, leveraging a solution like SmartSuite simplifies task management, data handling, and regulatory compliance, ultimately leading to a more effective CAPA process.
Adopting best practices and overcoming common challenges will further solidify the foundation for superior quality management in pharmaceutical operations.
Get started with SmartSuite Governance, Risk, and Compliance
Manage risk and resilience in real time with ServiceNow.



